
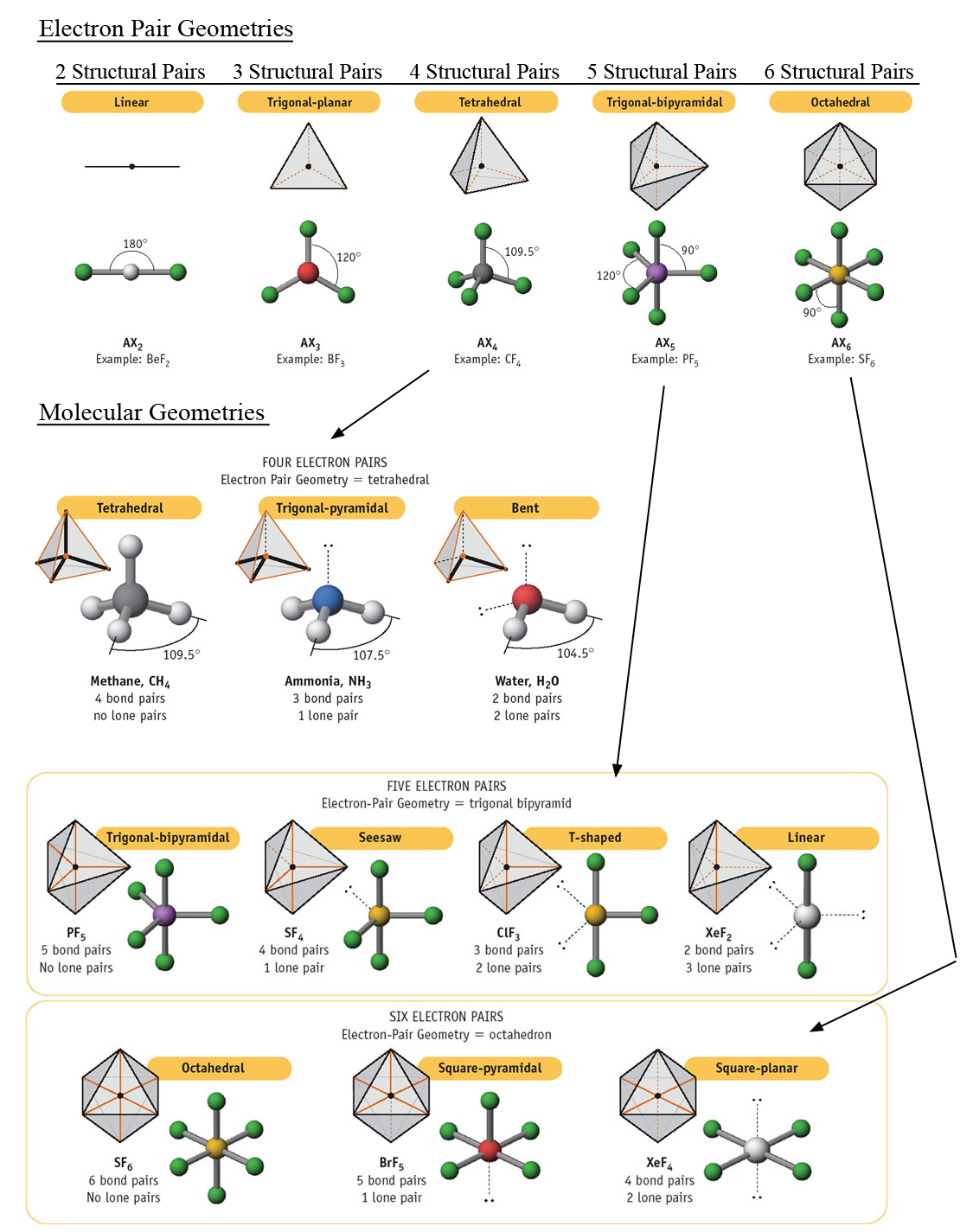
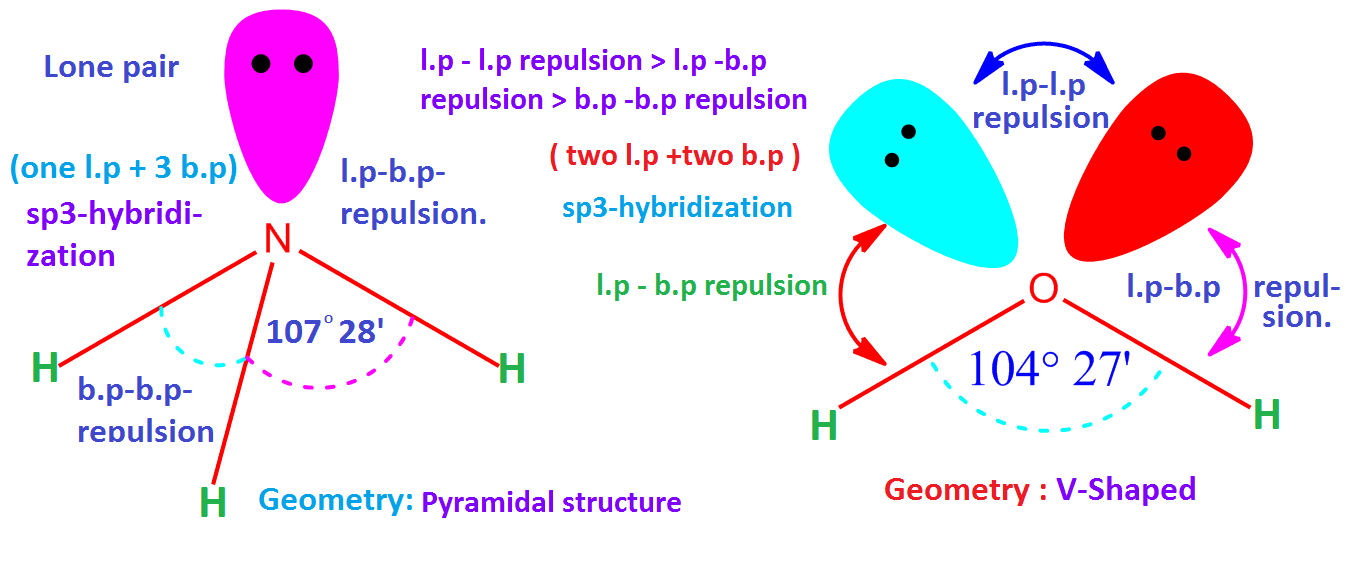

Kit includes 1 black carbon tetrahedral 9mm, 9 white hydrogen monovalent 9mm, 1 black carbon linear 9mm, 1 beige trigonal 9mm, 1 yellow sulfur octahedral 9mm, 6 light green fluorine monovalent 9mm, 1 purple phosphorus trigonal bipyramidal 9mm, 5 dark green chlorine monovalent 9mm, 1 yellow sulfur divalent 9mm, 2 red oxygen monovalent 9mm, 22 - 20mm grey straw bond. In this video we’ll use VSPRE Theory to practice the rules for identifying the major molecular geometries, including bond angles. The basic shapes depend on the steric number ( SN) of the central atom. What is the VSEPR theory used to predict VSEPR theory is used to predict the arrangement of electron pairs around central atoms in molecules, especially simple and symmetric molecules. Build the following molecules: Linear CO2, Bent SO2, Trigonal Planar BH3, Tetrahedral CH4, Trigonal bipyramidal PCl5, Octahedral SF6. What is the Vsepr shape of NCl3 So, according to the VSEPR chart, the electron geometry of NCl3 is tetrahedral while molecular geometry is trigonal pyramidal. Molecular Models 50 Piece Basic VSEPR Theory Molecule Shapes Models Kitīuild the following molecules Linear CO2, Bent SO2, Trigonal Planar BH3, Tetrahedral CH4, Trigonal bipyramidal PCl5, Octahedral SF6 | Build examples of the six basic VSEPR theory shapes with this economical and easy to assemble kit | Kit includes 1 black carbon tetrahedral 9mm, 9 white hydrogen monovalent 9mm, 1 black carbon linear 9mm, 1 beige trigonal 9mm, 1 yellow sulfur octahedral 9mm, 6 light green fluorine monovalent 9mm | Also includes 1 purple phosphorus trigonal bipyramidal 9mm, 5 dark green chlorine monovalent 9mm, 1 yellow sulfur divalent 9mm, 2 red oxygen monovalent 9mm, 22 - 20mm grey straw bondīuild examples of the six basic VSEPR theory shapes with this economical and easy to assemble kit.


 0 kommentar(er)
0 kommentar(er)
